It's the National Vaccine Program.
No, it's genocide.
It's a medical countermeasure.
No, it's a bioweapon.
It's legal! No, it's criminal!
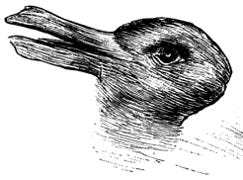
It’s a duck! It’s a rabbit!
It's both.
On June 9, 1969, Dr. Donald MacArthur testified to a US Senate hearing on DOD appropriations, about development of “new infective microorganisms which could differ in certain important aspects from any known disease-causing organisms. Most important of these is that it might be refractory to the immunological and therapeutic processes upon which we depend to maintain our relative freedom from infectious disease.”
Subsequent illegitimate, unconstitutional, pseudo-legislation passed by Congress and signed by US presidents purported to authorize and fund the American chemical and biological warfare and genocide program.
These laws addressed chemical and biological warfare and weapons testing programs; DOD reporting to Congress on chemical and biological weapons programs; judicial review; informed consent rights (for subjects) and obligations (for investigators) during human experiments; national emergencies; public health emergencies; terrorism; homeland security; HHS authority and program funding, research moratoria (including fetal tissue and genetic manipulation research); Posse Comitatus Act, Insurrection Act, domestic deployment of military against civilians; chemical and biological weapon stockpile management; strategic national pharmaceutical stockpile management; federal preemption of state and local laws; federal funding for state and local law alignment with federal medical-martial law programs; surveillance, quarantine, apprehension and detention powers; civil liability indemnification; Emergency Use Authorization/EUA products classified as medical countermeasures, covered countermeasures, security countermeasures, pandemic products, epidemic products; domestic propaganda; conduct of clinical trials, use of real-world evidence; Other Transaction Authority/OTA ‘prototype’ procurement DOD contracting with private companies to produce EUA products; mass testing programs; and DOD-HHS agreements to “provide support for vaccination programs...through use of the excess peacetime biological weapons defense capability of the DOD.”
Through this legislation, pseudo-authorized crimes have been pseudo-codified in the United States Code at Title 6 (Domestic Security); Title 10 (Armed Forces); Title 21 (Food and Drugs); Title 22 (Foreign Relations); Title 42 (Public Health and Welfare); and Title 50 (War and National Defense).
These pseudo-laws include: Armed Forces Appropriation Act (Nov. 19, 1969); National Cancer Act (Dec. 23, 1971); National Research Service Award Act (July 12, 1974); National Emergencies Act (Sept. 14, 1976); Department of Defense Appropriations Authorization Act of 1978 (July 30, 1977); Department of Education Organization Act (Oct. 17, 1979); 1982/12/21 - Congressional Reports Elimination Act (Dec. 21, 1982); 1983/07/13 - Public Health Service Act Amendment (July 13, 1983); Health Research Extension Act (Nov. 20, 1985); State Comprehensive Mental Health Services Plan Act/National Childhood Vaccine Injury Act/National Vaccine Program (Nov. 14, 1986); Health Omnibus Programs Extension Act. (Nov. 4, 1988); Robert T. Stafford Disaster Relief and Emergency Act. (Nov. 23, 1988); Omnibus Budget Reconciliation Act (Dec. 19, 1989); National Institutes of Health Revitalization Act (June 10, 1993); NDAA for FY1994 (Nov. 30, 1993); NDAA FY1996 (Feb. 10, 1996); Antiterrorism and Effective Death Penalty Act (April 24, 1996); NDAA FY1998 (Nov. 18, 1997); Food and Drug Administration Modernization Act (Nov. 21, 1997); NDAA FY1999 (Oct. 17, 1998); Omnibus Consolidated and Emergency Supplemental Appropriations Act FY1999 (Oct. 21, 1998); Public Health Improvement Act/Public Health Threats and Emergencies Act (Nov. 13, 2000); Authorization for Use of Military Force (Sept. 18, 2001); PATRIOT Act [Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism] (Oct. 26, 2001); Public Health Security and Bioterrorism Preparedness and Response Act (June 12, 2002); Homeland Security Act (Nov. 25, 2002); NDAA FY2004 (Nov. 24, 2003); Project Bioshield Act (July 21, 2004); Department of Defense, Emergency Supplemental Appropriations to Address Hurricanes in the Gulf of Mexico, and Pandemic Influenza Act/Public Readiness and Emergency Preparedness (PREP) Act. (Dec. 30, 2005); NDAA/John Warner Defense Authorization Act FY2007 (Oct. 17, 2006); Pandemic and All-Hazards Preparedness Act (Dec. 19, 2006); National Institute of Health Reform Act (Jan. 15, 2007); Food and Drug Administration Amendments Act of 2007 (Sept. 27, 2007); NDAA FY08 (Jan. 28, 2008); Patient Protection and Affordable Care Act/ObamaCare (March 23, 2010); NDAA FY2011 (Dec. 31, 2011); Food and Drug Administration Safety and Innovation Act (July 9, 2012); NDAA FY2013 (Jan. 2, 2013); Disaster Relief Appropriations Act (Jan. 29, 2013); Pandemic and All-Hazards Preparedness Reauthorization Act. (March 13, 2013); Medicare Access and CHIP Reauthorization (MACRA) Act (April 16, 2015); NDAA FY2016 (Nov. 25, 2015); NDAA FY2017 (Oct. 17, 2016); 21st Century Cures Act (Dec. 13, 2016); NDAA FY2017 (Dec. 23, 2016); FDA Reauthorization Act (Aug. 18, 2017); NDAA FY2018 (Dec. 12, 2017); Act to amend Food Drug and Cosmetics Act Emergency Use Authorization statute, 21 USC 360bbb-3 (Dec. 12, 2017); Federal Aviation Administration Reauthorization Act/Disaster Recovery Reform Act (Oct. 5, 2018); Pandemic and All-Hazards Preparedness and Advancing Innovation Act (June 24, 2019); Coronavirus Preparedness and Response Supplemental Appropriations Act (March 6, 2020); Families First Coronavirus Response (March 18, 2020); Coronavirus Aid, Relief, and Economic Security CARES Act (March 27, 2020); Paycheck Protection Program and Health Care Enhancement Act (April 24, 2020); Consolidated Appropriations Act (Dec. 27, 2020); NDAA FY2021 (Jan. 1, 2021); American Rescue Plan/Consolidated Appropriations Act (March 11, 2021); NDAA FY2022 (Dec. 27, 2021); Consolidated Appropriations Act (March 15, 2022).
MEANWHILE...
Congress has also been passing laws to comply with international treaties prohibiting crimes including genocide, biological weapons, torture, chemical weapons, war crimes and slavery, and protecting religious and civil liberties.
These laws have been codified in Title 18 (Crimes and Criminal Procedure) and include Genocide Convention Implementation Act of 1987 (Nov. 4, 1988); Biological Weapons Antiterrorism Act of 1989 (May 22, 1990); Religious Freedom Restoration Act (Nov. 16, 1993); Foreign Relations Authorization Act FY94 and FY95 - Torture Convention implementation (April 20, 1994); Chemical Weapons Convention Implementation Act of 1998 (Oct. 21, 1998); War Crimes Act - Geneva Conventions implementation (Aug. 21, 1996); Military Commissions Act of 2006 - Geneva Conventions implementation (Oct. 17, 2006); and Leahy-Smith America Invents Act/Section 33 prohibition on issuing of patents “directed to or encompassing a human organism.” (Sept. 16, 2011).
Many of these American laws are built with large pseudo-legal loopholes purporting to make crimes not be crimes if committed by administrative and military officers representing the US Government.
MEANWHILE...
American presidents have been signing pseudo-laws called Executive Orders, Proclamations, Declarations and Directives: Executive Order 12452 expanded list of communicable diseases subjecting citizens to forcible apprehension and detention under HHS Secretary quarantine authority (1983); EO 13139 forced experimental, FDA-unapproved vaccines on armed forces without informed consent (1999); Proclamation 7463 placed US population under "national emergency" due to "terrorist attacks," renewed annually since (2001); EO 13324 blocked property ownership and transactions with terrorists (2001); EO 13295 added symptomatic SARS to quarantinable communicable diseases (2003); EO 13375 added symptomatic influenza to quarantinable communicable diseases (2005); National Security Presidential Directive 51, US government continuity of operations policy (2007); EO 13546, Optimizing the Security of Biological Select Agents and Toxins in the United States (2010); EO 13674 added asymptomatic, suspected SARS to quarantinable communicable diseases (2014); EO 13747, Advancing the Global Health Security Agenda to Achieve a World Safe and Secure from Infectious Disease Threats (2016); EO 13859, Maintaining American Leadership in Artificial Intelligence (2019); and EO 13874, Modernizing the Regulatory Framework for Agricultural Biotechnology Products (2019).
EO 13887, Modernizing Influenza Vaccines in the United States to Promote National Security and Public Health, directed rapid-deployment mRNA/DNA/LNP/nanotech drugs and devices (2019); a Biden "directive" to DOD ordered COVID-19 vaccination added to list of required military injections (2021); SecDef Austin ordered force injection of US military (2021); EO 14042, ordered forced injection of federal contractors (2021); EO 14043 ordered forced injection of federal employees (2021); a Biden "directive" to Department of Labor ordered forced injection of employees at private companies with more than 100 workers; EO 14047 added measles to the list of quarantinable communicable diseases (2021); a Biden "directive" to Department of Health and Human Services ordered forced injection of health care workers; EO 14067, Ensuring Responsible Development of Digital Assets (2022); EO 14081, Advancing Biotechnology and Biomanufacturing Innovation for a Sustainable, Safe, and Secure American Bioeconomy (2022).
MEANWHILE...
The white-collar murderers at the the Department of Health and Human Services were tightening the legal death traps: US Department of Health, Education and Welfare, National Institutes of Health, National Cancer Institute Special Virus Program, Progress Report 8 (1971); US HEW-NIH, National Cancer Institute Special Virus Program, Progress Report 9 (1972); HHS-Food and Drug Administration Final Rule Protections for Human Subjects; Prisoners Used as Subjects in Research (1981); HHS-FDA Final Rule Protection of Human Subjects; Informed Consent (1981); HHS Interim Final Rule: Informed Consent for Human Drugs and Biologics; Determination that Informed Consent is Not Feasible (1990); 1991 Common Rule (1991); HHS Interim Final Rule - Human Drugs and Biologics; Determination That Informed Consent Is NOT Feasible or Is Contrary to the Best Interests of Recipients; Revocation of 1990 Interim Final Rule; Establishment of New Interim Final Rule (1999); HHS FDA Draft Guidance Re: Emergency Use Authorization of Medical Products (2005); HHS FDA Guidance: Gene Therapy Clinical Trials - Observing Subjects for Delayed Adverse Effects (2006); HHS FDA Guidance - Emergency Use Authorization of Medical Products (2007); HHS Interim Final Rule - FDA Exceptions or Alternatives to Labeling Requirements for Products Held by the Strategic National Stockpile. (2007); HHS FDA Workshop Summary: Medical Countermeasures Dispensing: Emergency Use Authorization and the Postal Model…
“At the workshop, participants noted that EUA has a broader use beyond enabling the use of an unapproved product or extending the use of an approved product to populations for which it was not approved. In particular, it can also be used to address labeling requirements and other challenges that arise because of constraints inherent in a public health response. ‘From a legal perspective, there are a lot of situations where EUA helps get past all those requirements,’ said [Susan E. Sherman, J.D., M.S., is a senior attorney with the Office of the General Counsel, HHS] ‘You can change the labeling. You can change the information. You can change the dosage. You can give it to populations for which wasn’t approved.’ ” (2009)…
…HHS FDA Guidance for Industry: Potency Tests for Cellular and Gene Therapy Products (2011); HHS FDA Guidance: Decisions for Investigational Device Exemption Clinical Investigations (2014); HHS FDA Guidance: Considerations for the Design of Early-Phase Clinical Trials of Cellular and Gene Therapy Products (2015); HHS FDA Guidance: Design and Analysis of Shedding Studies for Virus or Bacteria-Based Gene Therapy and Oncolytic Products (2015); HHS Final Rule - HHS Clinical Trials Registration and Results. 81 Federal Register 64981 (2016); HHS Workshop Summary - The Nation's Medical Countermeasure Stockpile: Opportunities to Improve the Efficiency, Effectiveness, and Sustainability of the CDC Strategic National Stockpile (2016); HHS FDA Guidance: Emergency Use Authorization of Medical Products and Related Authorities (2017); HHS Final Rule - Federal Policy for the Protection of Human Subjects (2017); HHS Final Rule - Control of Communicable Diseases Final Rule (2017); HHS FDA Guidance: IRB Waiver or Alteration of Informed Consent for Clinical Investigations Involving No More Than Minimal Risk to Human Subjects (2017); HHS FDA Guidance: Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices (2017); HHS Final Rule - Federal Policy for the Protection of Human Subjects: Six Month Delay of the General Compliance Date of Revisions While Allowing the Use of Three Burden-Reducing Provisions During the Delay Period (2018); Material Transfer Agreement signed between US Health and Human Services (HHS) National Institutes of Health (NIH) National Institute for Allergies and Infection Diseases (NIAID), led by Anthony Fauci, University of North Carolina coronavirus researcher and patent-holder Ralph Baric, and Moderna, for “mRNA coronavirus vaccine candidates developed and jointly owned by NIAID and Moderna.” (2019); HHS FDA Guidance: Real-World Data - Assessing Electronic Health Records and Medical Claims Data To Support Regulatory Decision-Making for Drug and Biological Products (2021); HHS FDA Guidance: Real-World Data - Assessing Registries to Support Regulatory Decision-Making for Drug and Biological Products (2021); HHS Interim Final Rule - Possession, Use, and Transfer of Select Agents and Toxins—Addition of SARS–CoV/SARS–CoV–2 Chimeric Viruses Resulting From Any Deliberate Manipulation of SARS–CoV–2 To Incorporate Nucleic Acids Coding for SARS–CoV Virulence Factors to the HHS List of Select Agents and Toxins (2021); HHS Final Rule - National Vaccine Injury Compensation Program: Adding the Category of Vaccines Recommended for Pregnant Women to the Vaccine Injury Table (2022)
CULMINATING IN COVID...
Through pseudo-legal acts beginning in January 2020:
2020/01/27 - US Secretary of Health and Human Services Determination that a Public Health Emergency Exists and declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of this novel coronavirus. In continuous force since then, most recently renewed Oct. 13 by HHS Secretary Xavier Becerra.
2020/03/01 - HHS Centers for Medicare and Medicaid Services (CMS) COVID-19 Emergency Declaration Blanket Waivers for Health Care Providers, creating legal conditions for hospital homicide protocols.
2020/03/13 - President Trump issued a Stafford Act declaration (under the 1988 Stafford Act), and signed Proclamation 9994 (under the 1975 National Emergencies Act), Declaring a National Emergency Concerning the Novel Coronavirus Disease (COVID–19) Outbreak. Renewed every year since, most recently by Biden in Feb. 2022.
2020/03/24 - HHS Secretary Alex Azar issued Declaration of Emergency Use
Authorization, declaring “that circumstances exist justifying the authorization of emergency use of medical devices, including alternative products used as
medical devices.”





You continue to outdo yourself!
Brilliant!
Another "Save to Disk," folks.
If Fauci could put all our heads in cages with flies that would kill us he would but he can't so he uses phamaceuticide instead. What's the difference between Jeffrey Dahmer, who tortured animals as do most serial killers before they move on to torturing, and killing people, and Fauci? Quantity, and Fauci isn't a cannibal, as far as we know, but like John Wayne Gacy he may have bodies buried in a crawl space in his home.